The Saliva Puzzle:
Saliva Production & Its Influence on Swallowing
Guest Authors: Joanne Yee, MS, CF-SLP & Nicole Rogus-Pulia, PhD., CCC-SLP
Editor: Karen Sheffler, MS, CCC-SLP, BCS-S of SwallowStudy.com
Aspiration pneumonia (AP) is one of the leading causes of death among older adults, ranking among the top 10 most common diagnoses leading to in-hospital mortality in a recent study.1 The presence of oropharyngeal dysphagia is widely considered one of the most important risk factors for AP.2 Many patients who suffer from dysphagia and its negative health consequences also experience significant changes to salivation, including patients treated for head and neck cancer, patients post-stroke, patients with autoimmune diseases (e.g., Sjogren’s syndrome), and patients with dementia. Saliva is a complex bioactive substance that plays an important role in mastication, speaking, swallowing, taste, oral hygiene, and oral tissue integrity. (See blog series on oral hygiene / oral care and aspiration pneumonia prevention).
In this blog, we want to provide Speech-Language Pathologists and other healthcare professionals with a guide to understanding the complexity of saliva. We will discuss saliva as it pertains to:
- healthy deglutition and oral health,
- populations that are impacted by salivary dysfunction,
- its relationship to swallowing and aspiration pneumonia, and
- future research directions.
What is Saliva?
Whole saliva (commonly referred to as saliva) is the mixture of specific salivary fluid as produced by individual glands. It is composed primarily of water (~99.5%), with the remaining properties being proteins (0.3%) as well as inorganic and trace substances (0.2%). Though saliva is often perceived to be a fluid substance, it exists in gaseous and gel phases as well, sometimes simultaneously.3
Many factors influence the composition of saliva and its flow rate, including:
- type and size of the salivary glands producing saliva,
- presence or absence of stimulation,
- diet,
- drugs,
- age, and
- physiological status.
Two different kinds of saliva assist in oropharyngeal health and function: stimulated saliva and unstimulated (also called resting) saliva.
- Stimulated saliva is produced before, during, and after eating in the presence of various sensory inputs (e.g., mechanical, gustatory, etc.). It may also be secreted as a reflex to input from the higher centers of the brain in response to an emotional reaction.
- Unstimulated or Resting Saliva is not produced in the presence of these apparent stimuli. Instead, it has two components that affect its production: spontaneous and continuous secretion, as well as reflexive secretions to the sensation of dryness of the oral mucosa.4
This complex process serves in preserving the health of the oral cavity, while also maintaining the moisture of the oral and pharyngeal mucosa.
Where is saliva produced?
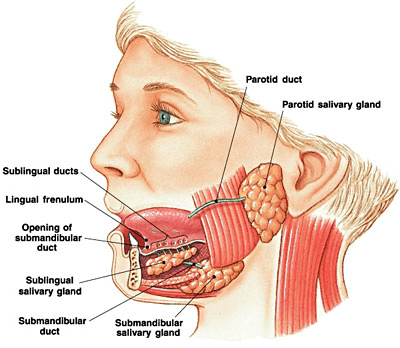
Saliva is produced in major contralateral glands in the oral cavity (pictured below), with the submandibular, sublingual, and parotid glands producing around 90-95 percent of resting saliva. The remaining 5-10 percent is produced by glands located throughout the oral mucosa, including the labial, buccal, lingual, and palatal mucosa. Each gland’s secretions show variable composition and volume and are dictated by the cellular make up of each gland.6
Image Source: https://entallergyandsinus.com/articles/the-throat/salivary-glands/
What is saliva composed of?
Each gland is made up of a mixture of cell types including acinar epithelial cells, of which there are two types: mucous cells and serous cells.
- Mucous cells produce secretions which are rich in mucin, a substance that is made up of proteins and amino acids, among other materials.7 Various types of mucins are produced and contribute to maintaining oral health in different ways, including lubrication and protection of the tooth surface.
- Serous cells secrete a watery fluid with comparatively few mucins and are strongly activated by stimuli.3
After being secreted by acinar cells, salivary fluid moves through the salivary ductal system picking up electrolytes and other proteins, before finally arriving at the excretory surface of the gland.7 The variation in composition of each gland characterizes the fluid it produces, affecting viscosity and elasticity4 as indicated below:
|
Major Gland |
Predominant Cell Type |
Secretion Type |
|
Parotid |
Serous |
Thin, watery, amylase-rich |
|
Submandibular |
Serous and mucous |
Viscous, mucin-rich |
|
Sublingual |
Mucous |
High viscosity, mucin-rich |
The viscoelastic properties and production volumes of these fluids contribute to various roles in oropharyngeal and digestive health. While watery serous fluid serves to moisten the oral mucosa, mucins aid in protective roles.
What are typical rates of salivary flow?
As noted above, many factors affect the amount and composition of saliva produced, including age, drugs, sex, circadian rhythm, sleep-wake cycles, and physiological status. As such, a large range of salivary flow rates is described to be within normal limits.
Total daily whole saliva flow may be between 500 mL and 1.5 L depending upon the individual, with average values for pH and flow rate for healthy individuals (i.e., with no reports of dryness or excessive salivation) as indicated in the following table.8
|
Unstimulated |
Stimulated |
|
|
Average whole saliva flow rate |
0.3 mL per minute |
7 mL per minute |
|
pH (on a scale of 0-14) |
6.2 (more acidic) |
7.4 (more basic) |
Studies have indicated that resting saliva’s pH and viscosity change more over the course of the day than stimulated saliva, which appears to be relatively stable (i.e., no effect seen for time of day). Although wide variability exists in terms of collection methods and stimuli used, evidence from the literature strongly suggests that a reduction in salivary flow is observed with aging. A recent meta-analysis by Affoo and colleagues5 demonstrated findings consistent with the present body of work in this area. The authors calculated that the flow rate of unstimulated whole saliva may decrease by about 40% in older subjects, whereas that of stimulated whole saliva may be reduced by about 15%. Interestingly, they observed a significant decrease in flow rates from the sublingual and submandibular glands, but did not observe similarly significant decreases in flow from the parotid or minor salivary glands.
(Editor’s Note: This is particularly fascinating. It may mean that an elder may have a decrease in the viscous and mucin-rich saliva. This may cause a perception of less lubrication, even though the visible quantity of the thin-watery saliva has not changed. We have to think not only about the quantity, but also the quality/chemistry of the saliva. More on this in the hyposalivation and xerostomia section below.)
What does saliva do?
Resting saliva
Resting saliva’s main function is maintenance of oropharyngeal health. It has been found to be 2-3 times more viscoelastic than stimulated saliva. In the absence of stimulation, the watery secretions of the parotid glands are thought to provide moisture to the mucosa, while the mucin-rich fluid of the sublingual and submandibular glands prevent dehydration as water evaporates due to breathing, swallowing of normal secretions, or absorption.9
Resting saliva also aides in protecting the surface of the teeth by clearing bacteria and by forming a protective layer on the surface. The structural properties of mucin are thought to cause microorganisms to entangle, clump together, and resist adherence to host tissues, whether that be the teeth or the mucosa. These microorganisms are then cleared by physical flushing of salivary flow and then swallowed. In addition, resting saliva forms a layer called the “salivary pellicle” on the surface of the teeth shortly after eating, which prevents erosion from bacteria and acid.10
Stimulated saliva
While resting saliva is composed of submandibular, sublingual, and parotid secretions, stimulated saliva is mainly composed of submandibular and parotid secretions. Notably, the parotid gland’s contributions in the presence of stimulation can increase to 50 percent of the volume of whole saliva produced.
Lubrication is one of the most important functions of stimulated saliva, as it prevents irritation of the oral mucosa and destruction of the tooth tissue (i.e., enamel) during speaking and deglutition. The mechanism by which lubrication occurs is complex and has been studied at length with no straightforward correlations being made between viscosity, protein adsorption (i.e., adhesion leading to creation of film) and lubrication.3 It is thought that there are multiple mechanisms that aid in reducing intraoral friction, including the presence of the salivary pellicle and the viscoelastic properties of saliva itself.
During deglutition, saliva prepares the food for swallowing by moistening, lubricating, and facilitating bolus formation.3 It also initiates breakdown of the bolus for digestion, through secretions rich in enzymes (e.g., amylase, which breaks down carbohydrates) and electrolytes. Saliva is thought to stimulate and affect taste sensitivity, in addition to maintaining the health of taste receptor sites on the oral tongue. Finally, it serves in protecting the oral cavity following food consumption by neutralizing food or bacterial acids through its low pH.4
Proteins and peptides
In addition to all of these functions, the proteins and peptides contained in saliva have been shown to have antiviral, antibacterial, and antifungal effects. However, the concentration of these proteins and peptides are not of a sufficient enough concentration to eliminate all of the 700 species of microorganisms present in the oral cavity (Read More: Take a Trip to the Lungs with 12 Bacterial Species). Research has suggested that good oral hygiene in conjunction with the antibacterial and antiviral properties, could maintain pathogenic oral flora at sufficiently low levels for oral health to be maintained and to reduce the risk of systemic infection.11
Salivary Dysfunction
As mentioned above, typical salivary flow rates are difficult to establish without making a comparison to flow rates for individuals suffering from salivary dysfunction. Dysfunction comes in many forms and may be characterized by:
- Hyposalivation: decreased production of salivary fluid
- Xerostomia: the sensation of oral dryness that may or may not be accompanied by hyposalivation
- Burning Mouth Syndrome: an idiopathic condition characterized by a continuous burning sensation of the mucosa of the mouth.
- Sialorrhea: hypersalivation or overproduction of saliva
Hyposalivation and Xerostomia
Hyposalivation can be described as an objective measurement of decrease in typical salivary flow rate. It may come about as part of the aging process, due to disease (especially of neurological or tissue-altering origin), due to radiation, or as a side effect of medication, such as anticholergenics or antipsychotics.
Patients with hyposalivation may experience the following problems:
- difficulty clearing food and bacteria from dental and oral surfaces;
- difficulty chewing, swallowing and speaking;
- increased caries (tooth decay or cavities);
- problems retaining dentures; and
- sensations of burning mouth or xerostomia.
A strong association has been observed between xerostomia and hyposalivation. Whereas hyposalivation is a measurable reduction in salivary flow from individual or all salivary glands, xerostomia is the subjective sensation of having dry mouth.
For individuals who suffer from xerostomia, a clinically significant decrease in flow rate has been observed, as normal residual volumes are slow to be replaced following mucosal absorption or evaporation.9 In addition, changes to the thickness of the salivary film covering palatal and mucosal surfaces may occur. These flow rate and thickness changes result in localized areas of dryness in the mucosa, particularly in the palate. A study conducted by Dawes and Odlum12 found that patients who experienced severe hyposalivation and reported sensation of very dry mouth had salivary flow reduced by 29%. Dawes9 suggested that in order for the xerostomia to be avoided, an unstimulated salivary flow rate greater than 0.1-0.3 mL per minute may be necessary.
Interestingly, there are cases where xerostomia occurs without a documented decrease in salivary flow.13–19 Reasons for this lack of association between xerostomia and hyposalivation are not clear20, but lubrication and hydration functions of saliva are hypothesized to relate to changes in the quality or composition of saliva rather than the quantity.13,20 Lubrication is a function of the rheological characteristics of the salivary films and the bulk components of saliva as well as their interplay.15
Treatment
A multitude of options exist for management of hyposalivation and xerostomia, though the strength of the evidence regarding several of these options is variable.
Treatment options include:
- topical preparations such as toothpastes, mouthwashes, gums, or moisturizers;
- drug therapy (e.g., pilocarpine or cevimeline);
- acupuncture;
- electrostimulation;
- biological therapy (e.g., infusion treatment);
- gene therapy; and,
- experimentally, stem cell transfer or consumption of vitamins during and after radiation therapy for cancer to mitigate salivary dysfunction.14
Behavioral management may include:
- increasing consumption of water throughout the day to provide moisture to the oral cavity;
- avoiding mouth breathing;
- using a humidifier in the bedroom and during winter months; and
- avoiding caffeine or alcohol, which have drying and diuretic effects.9
An extensive review conducted by Wolff and colleagues21 detailed the application and efficacy of the above mentioned treatments and highlighted the need for future research into these management options. Drug therapies have been observed to be effective in relieving symptoms of xerostomia or hyposalivation; however, other side effects (e.g., sweating, joint pain) and broad lists of contraindications are noted for these treatment options. (Read more: 8 Tips for Survivors of Head and Neck Cancer, which includes sections on oral care and dry mouth and How to Perform Effective Oral Care, which includes a section on mucositis).
Sialorrhea
Sialorrhea, or hypersalivation, is a comparatively less-studied domain than hyposalivation and xerostomia. Nevertheless, presence of sialorrhea has a significant impact on the populations of people who suffer from it. Also called drooling or ptyalis, sialorrhea is characterized by excess saliva in the mouth that extends beyond the lip margin. There are many clinical and functional impacts, including: social impacts (i.e., embarrassment or isolation), aspiration, skin breakdown, odor, and infection.
Sialorrhea may occur as an isolated phenomenon or in conjunction with:
- neurological disorders (e.g., Amytrophic Lateral Sclerosis or Parkinson’s disease),
- developmental disorders (e.g., cerebral palsy or Down’s syndrome), or
- as a side effect of medications (e.g., cholergenics),
It may also be caused by failure of the systems which help to control, clear, and remove saliva from the oral cavity (e.g., muscle incoordination).19 If sialorrhea occurs as a result of neurological illness, then decreased neuromuscular control may also result in impaired swallowing.23
Treatment
Management of sialorrhea ranges a spectrum of conservative to more invasive approaches.
- Invasive approaches may include surgery or radiation, which, although more permanent, have their own side effects.
- Conservative approaches may include:
- oral-motor exercises to strengthen musculature,
- intra-oral devices (e.g., palatal training devices),
- changes in diet or habits, or
- medical management (i.e., anticholinergic agents that work to decrease secretion or botulinum toxin injections).
While some medications have demonstrated effectiveness in small doses (e.g., glycopyrrolate), others have been demonstrated to be ineffective for older patients or have cognitive side effects. (Read More: Drug-Induced Dysphagia, which includes a discussion of anticholinergic medications and others that can cause dry mouth and cognitive deficits).
A recent review by Lakraj and colleagues23 discussed the use of botulinum injections for patients suffering from sialorrhea, highlighting the efficacy of the treatment and low incidence of side effects. Transient dysphagia was noted to be the most concerning of side effects; however, the authors noted that this effect generally appeared to resolve within two weeks and affected a small number of patients.23
Effects of Salivary Gland Dysfunction on Swallowing and Oral Health
As discussed previously, saliva plays a major role in oral health and swallowing. Given its importance, patients with salivary dysfunction may be at a greater risk for health problems such as aspiration pneumonia.
During deglutition and the oral phase of swallowing, saliva helps to lubricate the oral mucosal to decrease friction, while also moistening and creating cohesion of the bolus to prepare it for transit through the pharynx and esophagus. Results of studies designed to examine the influence of hyposalivation on measures of swallowing physiology have been conflicting.24–26 In several studies, lower salivary flow rates were found to be associated with longer oral phase durations27,28 and prolonged pharyngeal transit times29 during swallowing. However, other more recent studies showed no association between salivary flow rate and various measures of swallowing biomechanics.24,25,30 Kim and colleagues25 found that delayed oral swallow initiation reduced bolus viscosity and cohesion, particularly in substances which required additional masticatory effort. The authors also posited that patients with a history of stroke may need to chew food longer in order to achieve optimal food particle size, potentially as a result of insufficient masticatory ability, which they did not measure.
Increased effort in mastication and swallowing has been noted in the literature to have a significant impact on quality of life and nutrition; these are each affected by salivary dysfunction. In patients with hyposalivation, this effect has been measured by reports of food avoidance, low nutritional assessment score and anthropometric measures of malnutrition (e.g., measures of height and body weight to calculate body mass index) among other measures. One recent study conducted by Iwasaki et al in 2015 found that these patients had statistically significant higher percentages of self-perceived chewing and swallowing problems, frequency of diabetes, and decreased measures on the General Health Questionnaire, which included measures of anxiety and stress. These patients also reported reduced intake of proteins and vitamin B12, significantly reduced intake of fatty acids and important vitamins (including potassium, vitamin D, and vitamin E) and significantly reduced intake of vegetables as compared to the patients without hyposalivation. The authors reported that this epidemiological evidence demonstrates a deterioration in chewing ability, an avoidance of particular food groups (particularly vegetables), and overall poor nutrient intake.32
The diversity of bacterial flora which is present in the oral cavity is carefully balanced in healthy individuals. However, this balance can be altered by the presence of disease, malnutrition, or inactivity (e.g., non-oral diet / NPO status). Presence of oral or dental disease may increase or change the composition of oral flora, particularly as continued lack of oral hygiene contributes to an increase in the mass and complexity of dental plaque.33 This problem may be further compounded by a reduction of saliva as a result of decreased oral intake or as a side effect of medication. Reduction in flow may increase the concentration of bacteria in saliva, which if aspirated may be potent and pathogenic.
Additionally, lower salivary flow rates have been found to result in reduced swallowing frequency.34 A decrease in swallowing frequency can lead to less clearance of bacteria from the oral cavity.
For hospitalized or nursing home patients, the need for oral care assistance is recognized but effective oral hygiene is infrequently practiced and inadequate salivation is rarely addressed therapeutically. A recent study conducted by Foley and colleagues35 found that, for patients with dementia, the odds of pneumonia-associated mortality were increased more than 2 times than patients without dementia. These alarming results are likely due to some combination of poor oral hygiene, lack of adequate salivation, and the high prevalence of dysphagia in this patient population. (Read More about the impact of critical illness and poor oral hygiene on the oral microbiome in these blogs: What Do Volcanoes & Mouths Have in Common? and It’s Alive! Oral Microbiome).
Conclusions
In summary, saliva plays many critical roles in maintenance of oral health and efficient oral intake. The influence of inadequate saliva on swallowing function requires further study. A greater understanding of the complex relationships among saliva production, oral health, and dysphagia will inform the development of novel treatments that are aimed at minimizing the risk for pneumonia development in patients suffering from dysphagia. Clinically, it is important to measure the patient’s salivary function if it is suspected to be abnormal and to consider what existing treatment options may be available.
References
1. Le Guen M, Tobin A. Epidemiology of in-hospital mortality in acute patients admitted to a tertiary level hospital. Intern Med J. February 2016:Accepted January 2016. doi:10.1111/imj.13019.
2. DiBardino DM, Wunderink RG. Aspiration pneumonia: A review of modern trends. J Crit Care. 2015;30(1):40-48. doi:10.1016/j.jcrc.2014.07.011.
3. Schipper RG, Silletti E, Vingerhoeds MH. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch Oral Biol. 2007;52(12):1114-1135. doi:https://dx.doi.org/10.1016/j.archoralbio.2007.06.009.
4. Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. J Oral Rehabil. 2007;34(10):711-723. doi:10.1111/j.1365-2842.2007.01794.x.
5. Affoo, RH, Foley C, Garrick R, Siquiera WL, Martin RE. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J Am Geriatr Soc. 2015;63(10):2142-2151. doi:10.1111/jgs.13652.
6. Tabak LA, Levine MJ, Mandel ID, Ellison SA. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982;11(1):1-17. doi:10.1111/j.1600-0714.1982.tb00138.x.
7. Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016;70(1):11-25. doi:10.1111/prd.12116.
8. Humphrey SP, Williamson RT. A review of saliva: Normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162-169. doi:10.1067/mpr.2001.113778.
9. Dawes C. How Much Saliva Is Enough for Avoidance of Xerostomia? Caries Res. 2004;38(3):236-240.
10. Tabak LA. In Defense of the Oral Cavity: The Protective role of Salivary Secretions. Pediatr Dent. 2006;28(2):110-117.
11. Dawes C, Pedersen AML, Villa A, Ekstro¨m J, Proctor GB, Vissink A, Aframian D, McGowan R, Aliko A, Narayana N, Sia YW, Joshi RK, Jensen SB, Kerr AR, Wolff A. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol. 2015;(60):8 6 3 – 8 7 4. doi:https://dx.doi.org/10.1016/j.archoralbio.2015.03.004.
12. Dawes C, Odlum O. Salivary status in patients treated for head and neck cancer. J Can Dent Assoc. 2004;70(6):397-400.
13. Oral Health. NIDCR/CDC Dental, Oral and Craniofacial Data Resource Center; 2002. https://drc.hhs.gov/report/14_1.htm.
14. Nederfors T. Xerostomia and hyposalivation. Adv Dent Res. 2000;14:48-56.
15. Christersson CE, Lindh L, Arnebrant T. Film-forming properties and viscosities of saliva substitutes and human whole saliva. Eur J Oral Sci. 2000;108(5):418-425.
16. Sreebny LM, Valdini A. Xerostomia. Part I: Relationship to other oral symptoms and salivary gland hypofunction. Oral Surg Oral Med Oral Pathol. 1988;66(4):451-458.
17. Klestov AC, Webb J, Latt D, Schiller G, McNamara K, Young DY, Hobbes J, Fetherston J. Treatment of xerostomia: a double-blind trial in 108 patients with Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol. 1981;51(6):594-599.
18. Johnson G, Barenthin I, Westphal P. Mouth dryness among patients in longterm hospitals. Gerodontology. 1984;3(3):197-203.
19. Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc 1939. 1987;115(4):581-584.
20. Castro I, Sepúlveda D, Cortés J, Quest AFG, Barrera MJ, Bahamondes V, Aguilera S, Urzúa U, Alliende C, Molina C, González S, Hermoso MA, Leyton C, González MJ. Oral dryness in Sjögren’s syndrome patients. Not just a question of water. Autoimmun Rev. November 2012. doi:10.1016/j.autrev.2012.10.018.
21. Wolff A, Fox PC, Porter S, Konttinen YT. Established and Novel Approaches for the Management of Hyposalivation and Xerostomia. Curr Pharm Des. 2012;18(34):5515-5521. doi:10.2174/138161212803307509.
22. von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, Wray D, Brennan MT, Carrozzo M, Gandera B, Fujibayashi T, Navazesh M, Rhodus NL, Schiødt M. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2007;103(SUPPL.). doi:10.1016/j.tripleo.2006.11.010.
23. Lakraj AA, Moghimi N, Jabbari B. Sialorrhea: Anatomy, Pathophysiology and Treatment with Emphasis on the Role of Botulinum Toxins. Toxins. 2013;5(5). doi:10.3390/toxins5051010.
24. Logemann JA, Smith CH, Pauloski BR, Rademaker AW, Lazarus CL, Colangelo LA, Mittal B, MacCracken E, Gaziano J, Stachowiak L, Newman LA. Effects of xerostomia on perception and performance of swallow function. Head Neck. 2001;23(4):317-321.
25. Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Mittal B, Gaziano J, Stachowiak L, MacCracken E, Newman LA. Xerostomia: 12-month changes in saliva production and its relationship to perception and performance of swallow function, oral intake, and diet after chemoradiation. Head Neck. 2003;25(6):432-437. doi:10.1002/hed.10255.
26. Rogus-Pulia NM, Logemann JA. Effects of reduced saliva production on swallowing in patients with Sjogren’s syndrome. Dysphagia. 2011;26(3):295-303. doi:10.1007/s00455-010-9311-3.
27. Hughes C, Baum B, Fox P, Marmary Y, Yeh C-K, Sonies B. Oral-pharyngeal dysphagia: A common sequela of salivary gland dysfunction. Dysphagia. 1987;1(4):173-177. doi:10.1007/BF02406913.
28. Caruso AJ, Sonies BC, Atkinson JC, Fox PC. Objective measures of swallowing in patients with primary Sjogren’s syndrome. Dysphagia. 1989;4(2):101-105.
29. Rhodus NL, Colby S, Moller K, Bereuter J. Quantitative assessment of dysphagia in patients with primary and secondary Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(3):305-310.
30. Rogus-Pulia NM, Logemann JA. Effects of reduced saliva production on swallowing in patients with Sjogren’s syndrome. Dysphagia. 2011;26(3):295-303. doi:10.1007/s00455-010-9311-3.
31. Kim IS, Han TR. Influence of Mastication and Salivation on Swallowing in Stroke Patients. Arch Phys Med Rehabil. 2005;86(10):1986-1990. doi:10.1016/j.apmr.2005.05.004.
32. Iwasaki M, Yoshihara A, Ito K, Sato M, Minagawa K, Muramatsu K, Watanabe R, Manz MC, Ansai T, Miyazaki H. Hyposalivation and dietary nutrient intake among community-based older Japanese. Geriatr Gerontol Int. May 2015. doi:10.1111/ggi.12500.
33. Scannapieco FA, Shay K. Oral Health Disparities in Older Adults: Oral Bacteria, Inflammation, and Aspiration Pneumonia. Dent Clin North Am. 2014;58(4):771-782.
34. Rudney JD. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit Rev Oral Biol Med Off Publ Am Assoc Oral Biol. 1995;6(4):343-367.
35. Foley NC, Affoo RH, Martin RE. A Systematic Review and Meta-Analysis Examining Pneumonia-Associated Mortality in Dementia. Dement Geriatr Cogn Disord. 2015;39(1-2):52-67.
Meet the Guest Authors:
Joanne Yee, MS, CF-SLP
Joanne Yee received her master’s degree in speech-language pathology from the University of Wisconsin-Madison in May 2016. She holds a bachelor of arts degree in linguistics from the University of California, Santa Cruz. She is completing her clinical fellowship year at Mile Bluff Medical Center in Mauston, Wisconsin. She is interested in dysphagia across the lifespan.
Nicole Rogus-Pulia, PhD, CCC-SLP
Dr Rogus-Pulia is an Advanced Geriatrics Fellow at the William S. Middleton Memorial Veterans Hospital and an Adjunct Assistant Professor in the Department of Medicine at the University of Wisconsin-Madison. Clinically, Dr. Rogus-Pulia serves as Director of the multi-site Swallow STRengthening OropharyNGeal (Swallow STRONG) Clinical Demonstration Program funded by the Veterans Health Administration (VHA).
Dr. Rogus-Pulia completed her master’s degree in speech-language pathology at the University of Iowa; earned her doctoral degree from Northwestern University under the guidance of Dr. Jeri Logemann; and recently completed an Advanced Women’s Health Fellowship with Dr. JoAnne Robbins as her mentor.
The goal of Dr. Rogus-Pulia’s research program is to systematically identify and characterize factors underlying dysphagia in older adults and translate these findings into novel, evidence-based treatments for prevention of pneumonia onset. Her specific research interests include the effects of tongue strengthening on swallow function and health status, the impact of altered salivary production on swallowing, and the design of interventional studies for dysphagia in patients with dementia.